
Koselugo is a prescription medicine that is used to treat adults and children 1 year of age and older with neurofibromatosis type 1 (NF1) who have plexiform neurofibromas, also called plexiforms, that cause symptoms and cannot be completely removed by surgery.
Surgery is an effective treatment for NF1 plexiforms but may not be recommended by your doctor if your plexiforms are closely
intertwined with critical nerves and blood vessels, located near major organs, or cannot be completely removed by surgery.
Koselugo provides a treatment option for adults whose plexiforms cause symptoms and cannot be completely removed by surgery.
Ask your doctor if Koselugo is right for you.
Surgery is an effective treatment for NF1 plexiforms but may not be recommended by your doctor if your plexiforms are closely
intertwined with critical nerves and blood vessels, located near major organs, or cannot be completely removed by surgery.
Koselugo provides a treatment option for adults whose plexiforms cause symptoms and cannot be completely removed by surgery.
Ask your doctor if Koselugo is right for you.
Koselugo was proven to shrink plexiforms in a clinical study.
The KOMET study compared the effect of Koselugo on shrinking plexiforms with the effect of a placebo (treatment that doesn't contain medicine).
The KOMET study compared the effect of Koselugo on shrinking plexiforms with the effect of a placebo (treatment that doesn't contain medicine).
This study is still ongoing and continues to collect data on the long-term safety and effectiveness of Koselugo in adults.
The study included 145 adults with NF1 and plexiforms that could not be completely removed by surgery. The adults were split into 2 groups: 1 group received Koselugo treatment while the other received a placebo. After 12 months of treatment, all adults were given Koselugo.
The primary objective was overall response rate (ORR) after 16 months of treatment, which tells us how many patients saw their plexiform either disappear or shrink by 20% or more from the start of the study.
Other KOMET study objectives include:
The study included 145 adults with NF1 and plexiforms that could not be completely removed by surgery. The adults were split into 2 groups: 1 group received Koselugo treatment while the other received a placebo. After 12 months of treatment, all adults were given Koselugo.
The primary objective was overall response rate (ORR) after 16 months of treatment, which tells us how many patients saw their plexiform either disappear or shrink by 20% or more from the start of the study.
Other KOMET study objectives include:
- Time to response, or how long it takes for a person who achieved 20% or more plexiform shrinkage to see that shrinkage
- Duration of response, or the amount of time that people in the study who achieved 20% or more plexiform shrinkage maintained that shrinkage
- Safety information, including common side effects seen with Koselugo
Significantly more adults saw their plexiform shrink by at least 20% from the start of the study with Koselugo compared with placebo.
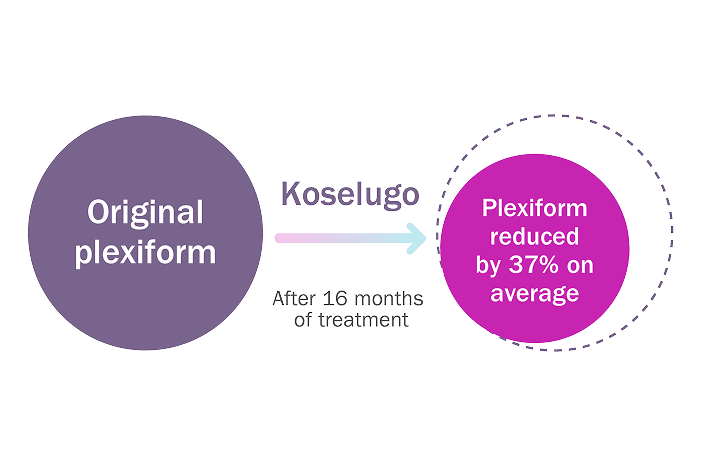
In adults whose plexiform shrank by 20% or more with Koselugo (n=14):
*A sustained effect is considered to be a response sustained for at least 6 months or more.
†The median time to response for adults taking Koselugo in the KOMET study was 3.7 months (range: 3.6 months to 11.2 months). The median is the middle point of how long it took adults to see their plexiform shrink by at least 20%. This means that half of the adults responded earlier and half responded later than 3.7 months.
‡In adults in the placebo group whose plexiform shrank by 20% or more, it is not known how long their plexiform remained reduced in size.
†The median time to response for adults taking Koselugo in the KOMET study was 3.7 months (range: 3.6 months to 11.2 months). The median is the middle point of how long it took adults to see their plexiform shrink by at least 20%. This means that half of the adults responded earlier and half responded later than 3.7 months.
‡In adults in the placebo group whose plexiform shrank by 20% or more, it is not known how long their plexiform remained reduced in size.
These are not all the possible side effects of Koselugo. Your doctor may change your Koselugo dose, temporarily stop, or ask you to permanently stop taking Koselugo if you experience certain side effects.
In the KOMET study:
Remember, the information on this page does not replace medical guidance or directions from your doctor. Always talk to your doctor about side effects and how to manage them.
IMPORTANT SAFETY INFORMATION
What are the possible side effects of Koselugo?
Koselugo may cause serious side effects, including:
Before taking Koselugo, tell your healthcare provider about all your medical conditions, including if you:
What should I avoid while taking Koselugo?
Avoid St John’s wort, grapefruit or grapefruit juice, and Seville oranges during treatment.
Most common side effects in children include: vomiting, diarrhea, increased level of an enzyme called creatine phosphokinase (CPK) in your blood, dry skin, redness around the fingernails, nausea, skin bumps that look like acne, fever.
Most common side effects in adults include: rash, diarrhea, skin bumps that look like acne.
These are not all the possible side effects of Koselugo. Call your healthcare provider for medical advice about side effects. You may report side effects to AstraZeneca at 1-800-236-9933 or at https://us-aereporting.astrazeneca.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
INDICATION
What is Koselugo?
Koselugo is a prescription medicine that is used to treat adults and children 1 year of age and older with neurofibromatosis type 1 (NF1) who have plexiform neurofibromas that cause symptoms and cannot be completely removed by surgery.
It is not known if Koselugo is safe and effective in children under 1 year of age.
Please see Patient Information and Instructions for Use in the full Prescribing Information for Koselugo (selumetinib) or at https://alexion.com/Documents/koselugo_uspi.pdf.
What are the possible side effects of Koselugo?
Koselugo may cause serious side effects, including:
- Heart problems. Koselugo can lower the amount of blood pumped by your heart, which can be severe. Your healthcare provider will do tests before and during treatment to check how well your heart is working. Tell your healthcare provider right away if you get any of the following signs or symptoms: persistent coughing or wheezing, shortness of breath, swelling of your ankles and feet, tiredness, increased heart rate.
- Eye problems. Koselugo can cause eye problems that can lead to blindness. Your healthcare provider will check your vision before and during treatment. Tell your healthcare provider right away if you get new or worsening vision changes, including: blurred vision, loss of vision, dark spots in your vision (floaters), other changes to your vision.
- Stomach, intestine, and mouth (gastrointestinal) problems. Koselugo can cause diarrhea, vomiting, nausea, and mouth sores. Diarrhea can be severe with Koselugo. Tell your healthcare provider right away the first time that you get diarrhea during treatment. Your healthcare provider may give you medicine to help control your diarrhea and may tell you to drink more fluids.
- Skin problems. Koselugo can cause severe skin rashes. Tell your healthcare provider if you get any of the following signs of skin problems: rash that covers a large area of your body, flat skin rash, raised bumps on your skin, skin bumps that look like acne, blisters, peeling skin, itchy rash, hair thinning or hair loss (alopecia).
- Increased level of an enzyme called creatine phosphokinase (CPK) in your blood and muscle problems. Koselugo can cause severe muscle problems. Treatment with Koselugo may increase the level of an enzyme in your blood called creatine phosphokinase (CPK), which may be a sign of muscle damage. Increased level of CPK in the blood is common during treatment and can also be severe. Your healthcare provider should do a blood test to check your blood levels of CPK before you start taking Koselugo and during treatment. Tell your healthcare provider right away if you get any of the following signs or symptoms: muscle aches or pain; muscle spasms and weakness; dark, reddish urine.
Before taking Koselugo, tell your healthcare provider about all your medical conditions, including if you:
- Have heart problems.
- Have eye problems.
- Have liver problems.
- Females who are able to become pregnant:
- Your healthcare provider should check to see if you are pregnant before you begin treatment.
- You should use effective birth control (contraception) during treatment and for 1 week after your last dose.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment.
- Males with female partners who are able to become pregnant:
- You should use effective birth control (contraception) during treatment and for 1 week after your last dose.
- Are breastfeeding or plan to breastfeed. It is not known if Koselugo passes into your breast milk. Do not breastfeed during treatment and for 1 week after your last dose. Talk to your healthcare provider about the best way to feed your baby during this time.
What should I avoid while taking Koselugo?
Avoid St John’s wort, grapefruit or grapefruit juice, and Seville oranges during treatment.
Most common side effects in children include: vomiting, diarrhea, increased level of an enzyme called creatine phosphokinase (CPK) in your blood, dry skin, redness around the fingernails, nausea, skin bumps that look like acne, fever.
Most common side effects in adults include: rash, diarrhea, skin bumps that look like acne.
These are not all the possible side effects of Koselugo. Call your healthcare provider for medical advice about side effects. You may report side effects to AstraZeneca at 1-800-236-9933 or at https://us-aereporting.astrazeneca.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
INDICATION
What is Koselugo?
Koselugo is a prescription medicine that is used to treat adults and children 1 year of age and older with neurofibromatosis type 1 (NF1) who have plexiform neurofibromas that cause symptoms and cannot be completely removed by surgery.
It is not known if Koselugo is safe and effective in children under 1 year of age.
Please see Patient Information and Instructions for Use in the full Prescribing Information for Koselugo (selumetinib) or at https://alexion.com/Documents/koselugo_uspi.pdf.
COVID-19=coronavirus disease 2019; NF1=neurofibromatosis type 1; PN=plexiform neurofibromas.
