STAY INFORMED ABOUT NF1 PLEXIFORM NEUROFIBROMAS
To receive the latest updates, tools, and tips about NF1 plexiform neurofibromas and Koselugo, please provide us with some information about yourself.

Sam, age 10, living with NF1 PN.
Sam is a Koselugo patient.
KOSELUGO® (selumetinib) is the FIRST FDA-approved oral medication proven to shrink neurofibromatosis type 1 (NF1) plexiform neurofibromas (PN) when they cannot be completely removed by surgery.
You may know Koselugo as selumetinib. Koselugo is the brand name for selumetinib. When you see either of these names, it's important to know that they are referring to the same medication.

The SPRINT study was conducted by the National Cancer Institute (NCI) to test Koselugo in children and teens who could not have their plexiform neurofibromas removed by surgery.*
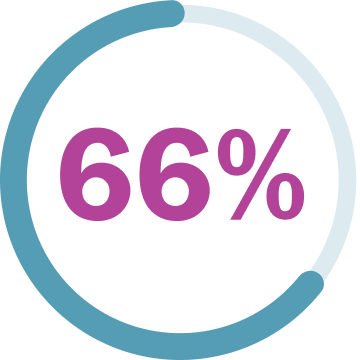
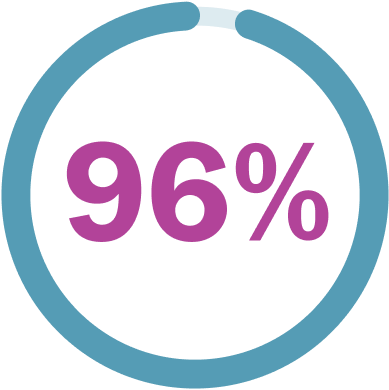
96% (48/50) of children and teens saw their PN shrink or stay about the same.**†
~2.5 years

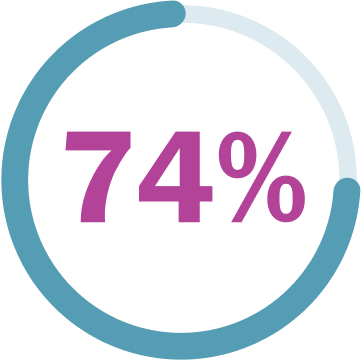
74% (37/50) of children and teens continued to see shrinkage or stability over 2.5 years later.‡





(26/33) maintained a PN reduction response for at least 2 years§


(21/33) maintained a PN reduction response for at least 3 years§
Your child's doctor may refer to this as “duration of response,” which is the amount of time that children and teens in the study achieved 20% or greater tumor shrinkage and maintained that shrinkage.
Watch a video about Kim and her son, Quentin, as they discuss his experience with Koselugo.
4+ YEARS
Koselugo has been prescribed at top NF1 centers across the country for over 4 years
*Confirmed by 3D magnetic resonance imaging (MRI) scan.
†The best response recorded as of June 2018 data cutoff. The median time to onset of response was 7.2 months (range: 3.3 months to 1.6 years). Median is defined as the middlemost point in a dataset.
‡One more person achieved 20% or greater PN shrinkage after about a year and a half.
§Data cutoff March 2021.
¶Data cutoff February 2021. This information is from the SPRINT long-term follow-up study.
In clinical trials, most children and teens had manageable side effects that did not require permanently stopping treatment.


In the SPRINT Phase 1 and 2 studies,# half of the children and teens took Koselugo for more than 4.4 years.|| Some children and teens in these studies are still taking Koselugo and the longest treatment period is yet to be determined.
In the Koselugo long-term follow-up study, no new side effects were identified.#**
(38/50) were able to stay on a full dose of Koselugo


(40/50) were able to avoid stopping treatment by temporarily pausing dosing


(44/50) were able to stay on treatment without permanently stopping due to a side effect
The most common side effects include:
These are not all the possible side effects of Koselugo. Your child’s doctor may change your child’s dose, temporarily stop, or permanently ask your child to stop taking Koselugo if they experience certain side effects.
Before and after starting treatment, dedicated specialists are available to offer personalized support for your family's needs.
Get supportTo receive the latest updates, tools, and tips about NF1 plexiform neurofibromas and Koselugo, please provide us with some information about yourself.
What are the possible side effects of Koselugo?
Koselugo may cause serious side effects, including:
Before taking Koselugo, tell your healthcare provider about all your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription, over-the-counter medicines, vitamins, or herbal supplements. Especially tell your healthcare provider if you are taking aspirin, blood thinners, or other medicines to treat blood clots. Koselugo contains vitamin E, which may increase risk of bleeding.
What should I avoid while taking Koselugo?
Do not drink grapefruit juice, eat grapefruit, or take supplements with grapefruit or St. John’s Wort during treatment.
Most common side effects include: vomiting, stomach-area pain, nausea, dry skin, muscle and bone pain, feeling of tiredness or lacking energy, fever, sores in your mouth, headache, redness around the fingernails, itching.
These are not all the possible side effects of Koselugo. Call your
healthcare provider for medical advice about side effects. Your
healthcare provider may change your dose, temporarily stop, or
permanently ask you to stop taking Koselugo if you have any of
these side effects.
You may report side effects to AstraZeneca at 1-800-236-9933 or
at
https://
us-aereporting.astrazeneca.com
or FDA at
1-800-FDA-1088
or
www.fda.gov/medwatch.
What is Koselugo?
All families shown in this website have been compensated by Alexion, unless otherwise noted.